Since hydrophilic groups such as a sulfonic acid group or a carboxylic acid group are present in the molecular structure of a dye such as a direct dye, a reactive dye, or an acid dye, their wet handling fastness is poor. At the same time, domestic sulphur dyes also have the disadvantage of low wet handling fastness, which limits their range of use. Although some imported dyes can make up for this deficiency to a certain extent, the effect of the fixing agent is still very important because of the high price and the unsatisfactory fastness of some dark varieties.
There are many kinds of commonly used fixing agents, which can be divided into three kinds according to their fixing route and structure [1]: (1) cationic surfactant type; (2) quaternary ammonium salt type without surfactant; (3) Resin reactive fixing agent. The resin reactive fixing agent has a certain solubility in water, is a pre-shrinkage with polyfunctional groups, can crosslink into a macromolecular network structure, can form a macromolecular compound with a dye, and is active in the epoxy group. The “bridge†between the fiber and the dye makes the dye and fiber more firmly bonded. Thereby improving the wet processing fastness. The fixing agent TX discussed herein belongs to this type. It can significantly improve the wet processing fastness of direct dyes and strong acid dyes, and hardly affects the color and feel of the fixing products, and can be stripped by conventional methods, and the dyeing property is good. Fixing agent TX uses formaldehyde-free raw materials, no formaldehyde is produced during the synthesis process, formaldehyde is not released after the fabric is fixed, and the synthesis method is simple and the raw materials are easy to obtain.
1 experimental part
1.1 Synthesis of Fixing Agent TX
In a four-necked flask equipped with a stirrer, a reflux condenser, a thermometer, and a constant pressure feeder, a solution of 1,5-dihydroxynaphthalene and diethyl ether was added, and a suitable amount of a boron trifluoride-diethyl ether complex solution was added thereto by a syringe to the reaction. The inside of the liquid; an appropriate amount of epichlorohydrin was added dropwise by a constant pressure feeder, and reacted at 40 to 60 ° C for several hours under stirring. Then, the temperature is lowered, and solid caustic soda is added to carry out a ring closure reaction. After completion of the reaction, the mixture was distilled under reduced pressure, and sodium chloride was filtered off, and the pH was adjusted to 6 to 7 with acetic acid [2, 3].
1.2 trait analysis
The obtained product is a reddish brown solid, soluble in ether and acetone, and has good water solubility. The product was identified by infrared spectroscopy analysis, and the structure contained a functional group having a designed epoxy value (mol/100 g) = [(V1 - V2) c] / 10 m.
The determination of the epoxy value by the hydrochloric acid acetone method, the standard method: accurately weigh the sample 0.5 ~ 1g (accurate to 0.0002g), placed in 250ml with a grindstone triangular conical flask, accurately added hydrochloric acid - 20 ml of acetone solution, densely packed. Shake well and place in a dark place, stand still for 30 minutes. Five drops of the mixed indicator solution were added, and the mixture was titrated to a purple-blue color with a 0.15 mol/L sodium hydroxide standard solution, and a blank experiment was performed.
Epoxy value (mol/100g) = [(V1-V2)c]/10m
Wherein the V1 blank sample consumes caustic soda volume/ml; the V2 sample consumes caustic soda volume/ml; c caustic soda standard solution concentration; m sample mass/g.
1.3 fixing effect test
The swatches dyed with direct dyes, acid dyes, and domestic sulphur dyes were post-treated with 2% (owf) fixing agent TX, and the various fastnesses were compared with the original samples. See GB3920-83, GB3921-83, GB3922-83 for experimental methods. At the same time, the fixing agent Y is used as a reference for comparison.
2 results and discussion
2.1 Synthesis Process Analysis
In the synthesis process, factors affecting the reaction include reaction temperature, reaction time, amount of catalyst, ratio of reactants, and the like. Through the screening of orthogonal experiments, the optimal synthesis conditions were determined: ring-opening reaction for 5 h, reaction temperature 50-55 ° C; ring closure reaction for 5 h, reaction temperature 30-35 ° C; catalyst dosage was 2% to 4% of the reactant; The material ratio is 1,5-dihydroxynaphthalene: epichlorohydrin: caustic soda = 1:2.5:2.
The non-ionic fixing agent TX is a prepolymer mainly composed of dinaphthol and epichlorohydrin, similar to the initial shrinkage of the resin, and its chemical structure:

Among them, the active epoxy group acts as a "bridge" between the fiber and the dye, reinforcing the combination of dye and fiber. Since the water solubility of the product decreases as the value of n increases, the reaction temperature, reaction time, and ratio of the reactants should be strictly controlled, otherwise the yield of the product will be affected.
2.2 Analysis of product identification results
From the infrared spectrum analysis of the starting materials and products, it can be seen that the influence of the reaction conditions on the progress of the reaction and the purity of the final product was changed. Among them, there are obvious epoxy peaks in the product spectrum under the optimal synthesis conditions (see Figure 1), which proves that the reaction is carried out in the designed direction [4]. The epoxy value of the product was determined by the hydrochloric acid acetone method to be 0.598 mol/100 g, and the theoretical value was 0.735 mol/100 g, thereby indicating that the number of units n>1, and the product was a mixture of various molecular weights.
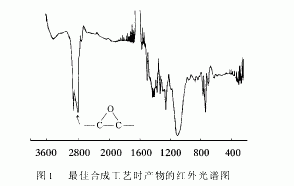
2.3 Fixing effect analysis
2.3.1 Fixation results of direct dye-dyed cotton fabrics (Table 1)

It can be seen from Table 1 that after the fixing agent TX treatment, the fastness of the direct dye can be improved by 1 to 2, indicating that the fixing agent TX has a significant effect on improving the wet processing fastness of the direct dyeing dyeing product. However, there are also deficiencies, such as the color light after fixing is slightly darker than the original.
2.3.2 Fixation results of acid dyed pure wool fabric (Table 2)
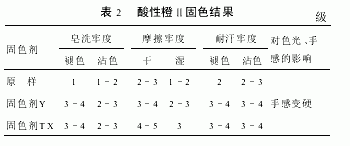
It can be seen from the experiment that TX has a strong fixing effect on the dyeing of strong acid dyes, which is a good promotion for the use of low-priced strong acid dyes.
2.3.3 Results of domestic vulcanized black dyed corduroy fixing treatment (Table 3)
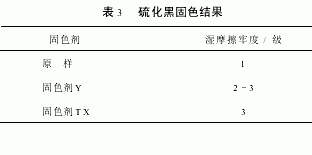
It can be seen from Table 3 that for the domestic sulphur black dye, the fixing agent TX can improve the wet rubbing fastness by 1 to 2 grades.
3 conclusions
3.1 From the structure of the reactants, the reaction history, TX is a new type of fixing agent that does not contain formaldehyde. It has a good fixing effect on direct dyes, strong acid dyes and sulphur dyes. Its fixing products can be used for export.
3.2 In the fixing process, different fixing agents should be used depending on the fixing type to achieve the best fixing effect. In addition, a small amount of citric acid or hexamethylene diamine can be appropriately added for use, and the fixing effect is better.
3.3 The fixing agent has a slight influence on the color light of the direct dye, and has little effect on the color light of the acid dye.
3.4 Since the fixing agent is nonionic, it can be used in the same bath as other nonionic, cationic or anionic additives.
3.5 The application mechanism of non-ionic formaldehyde-free fixing agent TX is currently under further study.
references
[1] Wang Cangcang. Introduction to textile dyeing and finishing additives [M]. Xi'an: Xi'an Jiaotong University Press, 1993.
[2] Yang Yongjun. Discussion on the synthesis process of glycerol epoxy resin [J]. Fine Chemicals, 1993, (4): 18.
[3] Zhao Guozhen, Yu Zeng. One-step synthesis of diglycidyl ether [J]. The Chemical World, 1990 (2): 6.
[4] Chen Yaozu. Organic analysis [M]. Beijing: Higher Education Press, 1983.
Galvanized Steel Pipe,Steel Pipe,Stainless Steel
Color Coated Steel Coil Co., Ltd , http://www.nssteel-coil.com